Heat Capacity of Calorimeter
The definition of the calorie is based on the specific heat of water defined. Heat Flux DSC comprises the sample and reference holder the heat resistor the heat sink and the heater.

Specific Heat Of A Metal Lab Youtube Science Chemistry Chemistry Class Chemistry Labs
Specific heat capacity is the most useful quantity available from DSC because it is directly related to sample properties and.

. Clamp the thermometer into the smaller hole with the stirrer. Heat capacity ratio of heat absorbed by a material to the temperature change. An alternative truly measuring at operational conditions lies in using an adiabatic flow calorimeter and thus evaluating the enthalpy balance of a small quantity of the HTF in a bypass of the system 5.
It is usually expressed as calories per degree in terms of the actual amount of material being considered most commonly a mole the molecular weight in grams. Place the immersion heater into the central hole at the top of the calorimeter. All data Tsonopoulos and Ambrose 1995.
The heat capacity in calories per gram is called specific heat. Place one litre 1 kg of water in the calorimeter. Block diagram of Heat Flux DSC.
Soc 1929 51 2738. Figure 1 shows the block diagram of Heat Flux DSC as an example. The heat capacity of toluene from 14 deg K to 298 deg K.
Heat Flux Type and Power Compensation Type. The entropy and the free energy of formation J. DSC is a commercially available instrument which has two 2 types.

Basic Calorimeter For Measuring Heat Capacity Is A Foam Coffee Cup Calorimeter Heatcapacity Coffee Cups Heat Heat Transfer
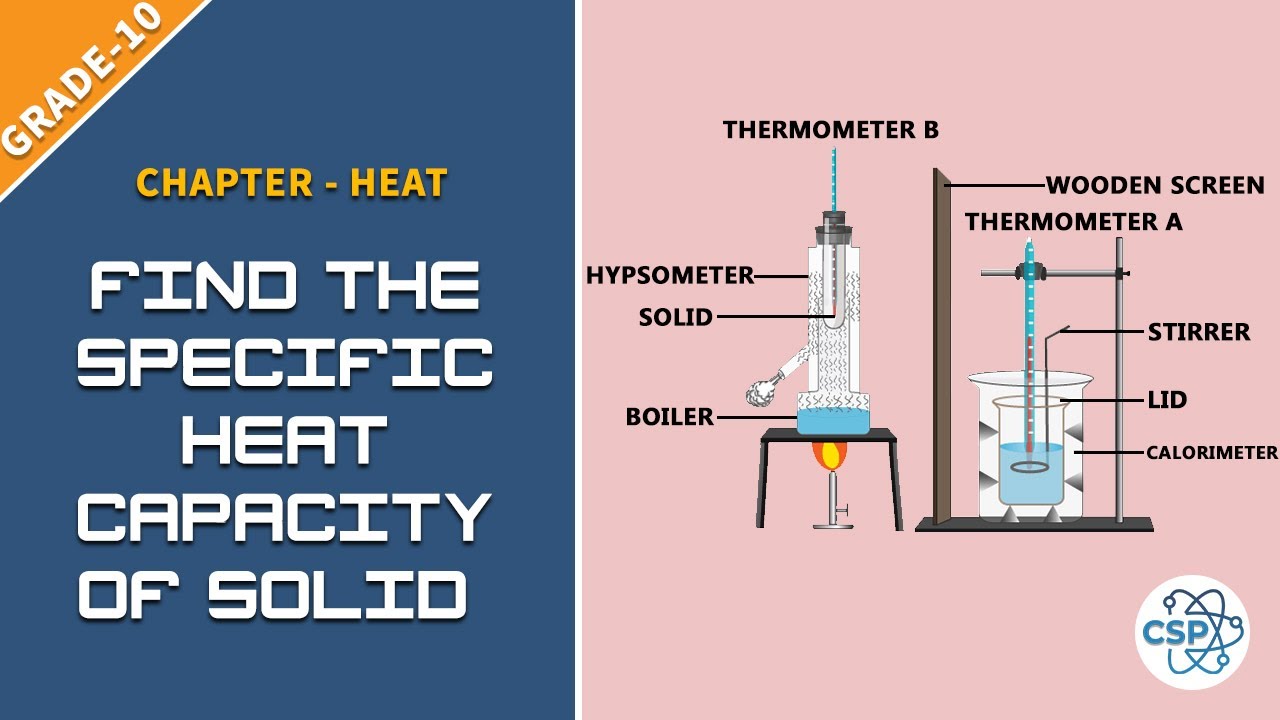
To Find The Specific Heat Capacity Of Solid By Using Method Of Mixtures See Class 10 Physical Properties Heat Science Experiments

Constant Volume Calorimetry For More Precise Work Than The Coffee Cup Calorimeter The Heat Capacity Of The Coffee Cups Chemistry Education Chemical Reactions

Pin By Redacted On Chemistry Education Chemistry Education What Is Science Ap Chem
No comments for "Heat Capacity of Calorimeter"
Post a Comment